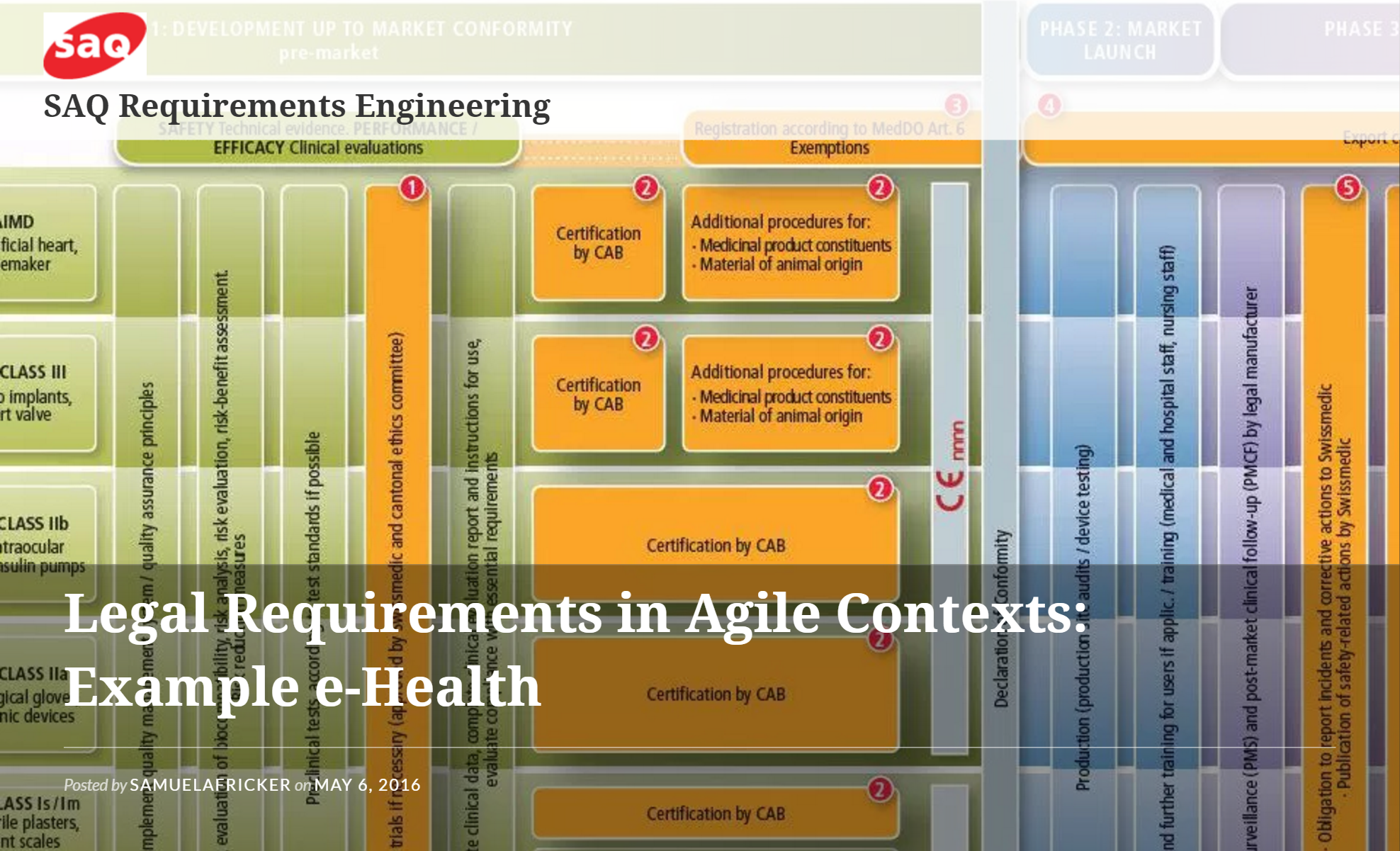
Regulations are an important mechanism to protect users from unsafe or otherwise dangerous products. At the same time, regulations inhibit creativity and innovation, especially of small and agile organizations with little experience in working with legal requirements. It is difficult to identify all regulations that are required for a product’s market-readiness and understand how the often vague and conflicting obligations should be adhered to.
In this session, we discuss what must and can be done for developing and working with legal requirements. To work with concrete examples and be relevant, we put the session into the context of agile development and e-Health. We take the perspectives of the regulatory body, process management platforms that promise compliance, and product organizations with experiences in achieving market-readiness.
Regulations are an important mechanism to protect users from unsafe or otherwise dangerous products. Learn and discuss first hand experience about agile development and e-Health.
PEDCO is engaged to foster scaled agility within regulated environments. We co-invite you to a free discussion session, held under the umbrella of the Swiss Association of Quality (SAQ) on June 2nd 2016 18:00 – 20:15 at Zurich Main-station. The chairman of this session is Prof. Dr. Samuel A. Fricker. Peter Pedross, our CEO and president of the board for computer science at SAQ will be a speaker for process management and scaled agility in regulated environments.
Speaker regulatory body: Swissmedic
Speaker process management platforms: PEDCO
Speaker market-readiness experiences: TBC
Light catering and beverages offered by SAQ.
Contact: Samuel Fricker