Key takeaways
- Learning and Benefits of such an approach.
- Be very clear on what is compliance and what is not.
- Compliance is often a ‘negotiation game’
- Demonstrate compliance in small iterations based on flow’
- Separate ‘What shall be done’ from ‘How something is done’
- You are not allone! Read/use available sources; e.g:
- A Lean QMS helps not only to establish and prove compliance, but also accelerates transformation and support a relentless improvement on an organizational level.
- This is nut just a simple promise, read our case studies.
Meet your Expert
CEO & Founder PEDCO AG,
- Chief Methodologist Applied SAFe
- Creator of the product Applied SAFe.
- Started to program for money at the age of 14. Ex-professional sportsman with a passion for software and a knack in engineering.
- 30+ years working experience with 50+ publications.
- SAFe SPC since 2011, Agile (XP) since 1999.
- Happy father and married to a wonderful woman.
Teaser
Learn about some lessons we learned! What are some typical bear traps? What does it need to reach your goal without producing waste? Where to find help? What are the benefits of such an endeavor?
Episode length: 6:30
Transcript with pictures:
There are several things we have learned from dealing with regulated environments in different industries.
Be very clear on what is compliance and what is not.
- Mapping of scaled agility to reference model is surprisingly straight forward, once the lean-agile mindset is understood
- Avoid the bear trap: Compliance elements shall always be mapped to value adding deliverables; instead of work products just produced to fulfill a requirement
- Define clearly the deliverables (min one, and value adding) to each activity
- Having good, automated mechanisms to prove mappings to reference models are needed to reduce discussion time and interpretation games
- Some reference models ask for process- and product-specific requirements -> Scope those requirements for purpose
Compliance is often a ‘negotiation game’
- Don’t forget the human element of stakeholders and appraisers;
Understanding of ‘How we do compliance’ is often based on a long legacy of deeply engrained expectations and patterns. - Solution Intent and agile Design control (e.g. Emergent design, skeleton approach) requires that participants really understand what they are doing
Demonstrate compliance in small iterations based on flow’
- Avoid ‘quality depth’ where compliance is ‘built in’ at the end of development.
- Treat audits like a normal system demo
- Focus on outcomes
Separate ‘What shall be done’ from ‘How something is done’
- Model only necessary steps in the process
- Build and rely on heuristics to model the process for usage, e.g.:
- Each activity has only one responsible role
- Each activity has always an output
- Activities with no inputs are very suspect
- Deliverables (I/O) are usually used more than just once
- Activities can be performed with different practices
- Ensure that practices can be changed/selected easily by performers
Quality Management System
- Let the QMS be easily accessible and easy to use.
- Promote transparency for each endeavor, Process instantiations need to be specialized in order to reduce waste.
- Allow fast process changes and pilot in appropriate levels.
- Allow process users to perform tailoring themselves in a controlled and easy way. Trust that they will do it good!
Your are not allone! Read/use available sources; e.g:
- Software Engineering Institute: ‘Scaling Agile Methods for Departments of Defense Program
- CMMI Institute: ‘A Guide to Scrum and CMMI’
- TIR45 from AAMI: ‘Agile practices in the development of medical device software’
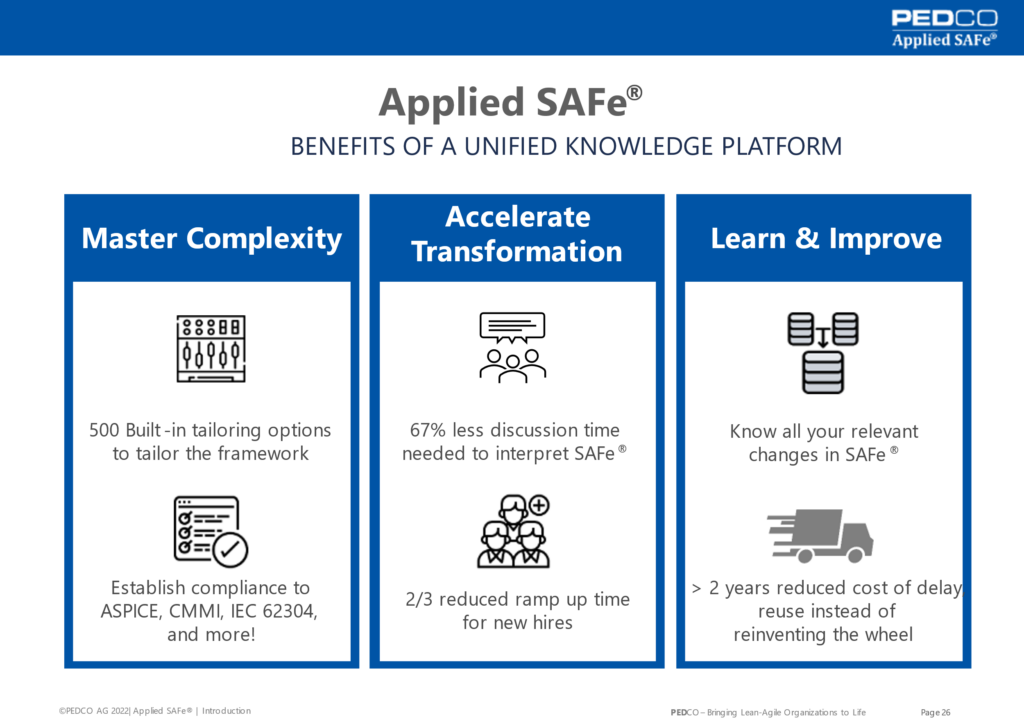
Something that we have also learned is that having such a lean QMS in place helps you to get these major benefits.
First it will allow you to master complexity and to ensure compliance to various regulated requirements.
Secondly and I think the most important, is that it will accelerate your transformation to towards a lean agile organization. According to feedback from our customers, they spent dramatically less discussion time to interpret safe and to ramp up time for new hires is reduced by up to 2/3.
Third thing is that you will learn and improve relentlessly. There will be so many new learnings and having such a system in place it’s very easy to track show relevant changes for every practitioner.
If you want to hear more about that or read our case studies just visit our web page or get in contact with us. Thank you so much we’re staying with me till the end of this serie of ‘PI planning in regulated environment.
Have a nice day and goodbye!
All episodes:
- Trailer – What you will get in this series –
- Compliance & Agile – Most regulatory requirements have a common base.
- Forget compliance, just follow the process – Relieve practitioners from the burden of compliance
- Regulatory requirements are our friends – Treat regulatory requirements as our friends and use them, to improve the processes.
- How to enhance SAFe for regulated environments – We showcase a concept and some examples.
- Lessons Learned – How to avoid bear traps and learn from each other.