As we have a lot of experience in scaled agile development in regulated environments, we are now actively expanding into medical device development. We already published a blog about TIR45 and agile practices in the development of medical device software how to apply scaled agility with our product Applied SAFe. These recommendations have been largely applied in our product Applied SAFe and allows customers to profit fast and precise from the benefits of agile development.
Meet us at the Medtec Europe 2018 in Stuttgart, Germany 17-19 April and discuss with us how to apply scaled agiltiy in medical device development with our product Applied SAFe.
Present at the booth will be Peter Pedross, CEO & Founder of PEDCO AG, as well as Dr. Marion Lepmets. We are looking forward talking to you!Register for free to join us at this conference!
Driving Opportunity and Innovation for the Medical Technology Industry
Medtec Europe is the preeminent medical technology platform showcasing the key trends and insights across the entire supply chain of the medical technology industry. It also serves as a prime location for business opportunities and start-up companies looking to present their innovations to investors.
The event brings together companies in the medical device supply chain to share ideas, meet partners, and discover, experience and source products & services from all stages of production in order to create the next generation of medical devices. Held annually, the show’s location is taking place in the great city of Stuttgart, Germany. Meet leading manufacturers, suppliers, and distributers and discover the latest product innovations from all over the world!
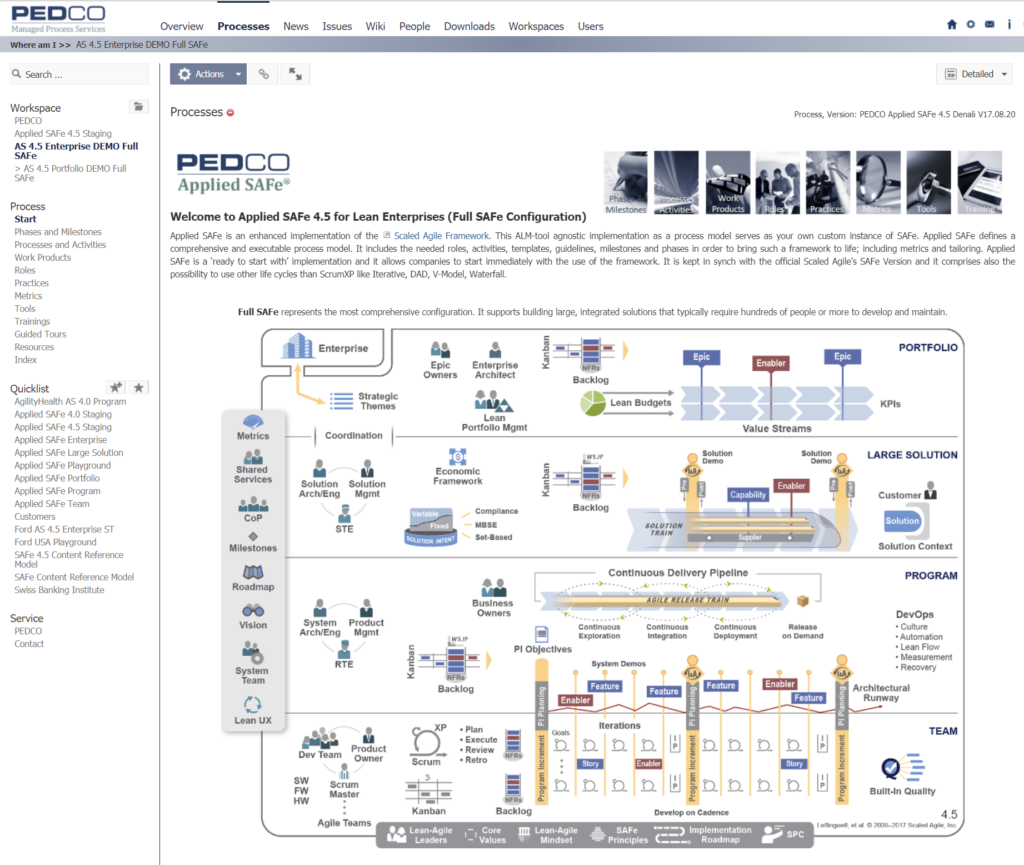
What is Applied SAFe?
Applied SAFe® is a fully fledged implementation of the Scaled Agile Framework (SAFe®) as a process model. It serves as your own customizable instance of SAFe®. Applied SAFe® is implemented as a comprehensive, executable process model. It includes all roles, activities, templates, guidelines, milestones and phases as defined by the SAFe® methodology in order to bring the framework to life; including metrics and tailoring. Applied SAFe® is a ‘ready to go with’ implementation and allows organizations to start using the framework almost immediately.
Applied SAFe® is a stand-alone product from PEDCO and is fully based on and kept in sync with the actual SAFe® version by Scaled Agile Inc. (SAI). Each release is also officially approved by SAI. As required, you can easily tailor all elements of the process model to your needs, simply by answering a series of out-of-the-box tailoring questions. It is also possible, to enhance and/or adapt the tailoring questions to create your own version. When moving to future versions of SAFe®, Applied SAFe® will automatically support you in the upgrade process and let you selectively decide whether to update your customized content to the latest “official” version or whether to keep your own. In short, Applied SAFe® allows you to extend or modify all of its content in order to better reflect the specific requirements of your organization.
Applied SAFe® can further include and ensure compliance with various reference models like CMMI, AS9100, CTR-180 (FDA), ISO9000 or your own company specific reference models. If you do not want to do this customization by yourself, we will help you to adapt the standard process implementation to your needs.
On your right you see the entry-page on the enterprise level just to give you a first impression of our product.
AGILE PRACTICES IN THE DEVELOPMENT OF MEDICAL DEVICE SOFTWARE
We already emphasized the importance and practicability of applying scaled agility in Medtec. Our product Applied SAFe enables directly to establish practices as described in the Technical Information Report #45(TIR45) by the ‘Association of Advancement of Medical Instrumentation’ [AAMI TIR45:2012]. This paper lays out the agile manifesto, several practices (e.g. Definition of Done), its application and tailoring in relation to reference models like IEC 62304, ISO 13485, ISO 14971 and ‘FDA CFR, Title 21, Part 820.30’. Please find below some excerpts of this paper.
AGILE was developed in response to quality and efficiency concerns posed by existing methods of software development. It can bring benefits that are valuable to the medical device software world, including the following:
- Continuous focus on safety, risk management, and delivering customer value through BACKLOG prioritization, planning practices, and customer feedback
- Continuous assessment of quality through continuous integration and testing
- Continuous improvement of the software development process through RETROSPECTIVEs and team accountability
- Continuous focus on “getting to DONE” and satisfying quality management stakeholders through the regular completion of activities and deliverables
AGILE can bring value to medical device software.
There are concerns about AGILE’s compatibility with the regulated world of medical device software development. For example, the AGILE Manifesto has value statements that seem contrary to the values of a quality management system; and because AGILE initially grew from the information-technology space where human safety and risk management were not of primary importance, there is concern that AGILE lacks the proper controls for producing safety-critical software.
Fortunately, AGILE‘s fundamental nature is to be adaptable to the context in which it is applied, allowing for AGILE principles and practices to be applied in ways that are compatible with the needs of the safety-critical, medical device software world.
AGILE can be adapted to the unique needs of medical device software.
This TIR will examine AGILE‘s goals, values, principles, and practices, and provide guidance on how to apply AGILE to medical device software development. It will
- provide motivation for the use of AGILE;
- clarify misconceptions about the suitability of AGILE; and
- provide direction on the application of AGILE to meet quality system requirements.
Following the guidance provided by this TIR can help medical device software manufacturers obtain the benefits provided by AGILE and satisfy regulatory requirements and expectations.
INITIAL RECOMMENDATIONS
This TIR provides recommendations for ways to effectively apply AGILE to medical device software. Here are some of the initial recommendations that are explained further later.
AGILE is driven by the value statements written in the Manifesto for AGILE Software Development. These value statements can seem to be contradictory to the values of the regulated world of medical device software, but they need not be interpreted that way. Instead, they can be aligned to enhance the effectiveness of the quality management system.
Apply the values of AGILE in a way that enhances a robust quality management system.
AGILE emphasizes the need for the team to own its practices, inspect them, adapt them, and optimize them to their context. Regulatory requirements emphasize the need to establish a robust quality management system. Within the context of an established quality management system, AGILE practices can be applied without disrupting the quality system and without raising undue concern among regulators.
Apply the practices of AGILE within the context of an established quality management system.
AGILE embraces a highly INCREMENTAL/EVOLUTIONARY lifecycle for software development. Although regulations and standards do not mandate a particular lifecycle model, if stakeholders have expectations for linear lifecycle models, an INCREMENTAL/EVOLUTIONARY lifecycle might bring challenges.
Set the correct expectations by defining the SOFTWARE DEVELOPMENT LIFECYCLE MODEL.
Demonstrate how an INCREMENTAL/EVOLUTIONARY lifecycle satisfies regulatory requirements.
As part of its INCREMENTAL/EVOLUTIONARY lifecycle, AGILE emphasizes the ability to respond quickly to change. Because rapid change can increase risks to product quality, effective change management systems are essential to align the desire to change quickly and the need to manage risk.
Establish robust change management systems to manage changes and mitigate risks associated with rapid change.

Meet us at Medtec!

Peter Pedross, CEO – PEDCO AG

Dr. Marion Lepmets, CEO
PEDCO Partner – Softcomply
PEDCO is proud to have one of the co-authors of this TIR 45 publication, Dr. Jochen Jäger, among its advisory board.

Dr. Jochen Jäger
Quality functional lead at:
Roche Diagnostics
Why Medtec?
Meet Europe’s leading medical device suppliers and Healthcare Professionals under one roof and review the latest product innovations from all over the world!
Every edition, Medtec Europe presents you with the latest updates and connects you with the world’s thought leaders of the medical device industry. Make use of the Medtec features like the Breakfast Meetings to network with decision makers, or the Medtec Innovation Tours, that lead you to the real significant innovations of the diverse Medtech product landscape.
Take a look at the video here to see the highlights from Medtec Europe 2017.