A lean quality management system can establish trust, alignment and in particular relieve the regulatory compliance in Enterprise Solution Delivery. Which skillset is required and what is a good platform to start pragmatically? How to connect product quality, lean quality management, and value streams? How to establish business agility in a large company?
Process quality has a significant impact on the overall quality mindset of an organization and is a booster for each and every agile transformation. Together with the Swiss Association of Quality (SAQ) we organize this webinar where we discuss together with you pro’s and con’s of different approaches. Examples will mostly come from Automotive, SAP and Aerospace-Industry.

Thomas Karl from Accenture and Peter Pedross from (CEO PEDCO and president of computer science at the Swiss Association of Quality) will talk about applied Lean Quality Management in a SAFe enterprise. During the presentation, we will uncover the hidden champion, in order to reach business agility.
Participants are kindly requested to join the discussion as well!
Register now!
https://us06web.zoom.us/webinar/register/WN__HL5TXOtRLSIEiu001J9-Q
When? Wed, November 10th, 2021 17:00 – 18:00 CET
This webinar will be held in English, questions can also be raised in German or French.
About this webinar:
At first glance, the practices associated with Lean-Agile and those associated with traditional quality management appear to be diametrically opposed, with conflicting goals and disparate communities. Through rigorous, stage-gated activities, the compliance world emphasizes quality, safety, and security to ensure that systems perform their intended purpose. Those systems demonstrate adherence to specifications through verification and validation (V&V) activities, reviews, audits, and sign-offs. To this community, change and variability equal added risk and uncertainty. By contrast, Lean-Agile development strives to discover the ultimate and optimal system iteratively, by creating an environment for learning. Building a working system in frequent, small batches confirms or rejects design hypotheses. Continuous customer/stakeholder collaboration provides fast feedback on decisions and the ability to adapt to new knowledge. Validated learning explores alternatives and helps ensure development creates products that meet the needs of customers. To this community, change and variability provide the ability to create products that excite customers and generate better economic results for the business
Software plays an increasingly important role in regulated environments. The principles of the agile manifesto are known to all Agilist, and although an overarching set of principles for regulated environments does not exist, a number of core issues for software development in regulated environments may be inferred. These issues include quality assurance, safety and security, effectiveness, traceability, and verification and validation.
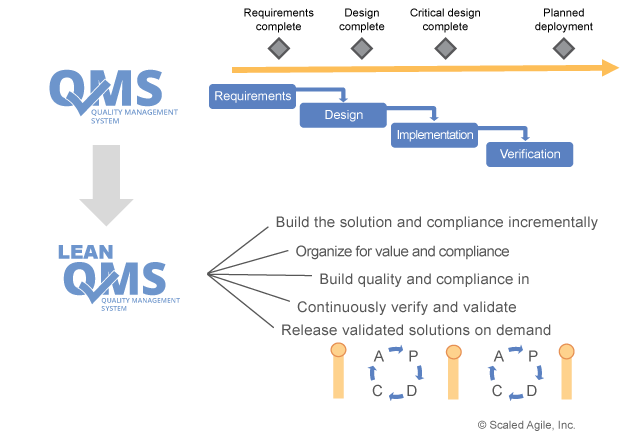
The picture on the right from SAFe® states that also the QMS needs to be Agile as well and in our case, need to take different life cycles into consideration. So, you can expect that the QMS itself will undergo much more changes than in the past. Fortunately, most lifecycles models describe more the organizational aspect of work (how to plan, improve, build, document etc.) but not as much about how something is done. So, you need to show how to synchronize milestones and different cadence of deliveries. A lot of companies have even milestones with numbers! Just imaging, what means milestone #7 if you are new to the company?
For example you need to be able to answer what happens with existing documentation. What kind of documentation is expected in regards of solution to be built/changed/maintained?
Regardless of the chosen lifecycle model and the sequence of activities, it is important to ensure that inputs and outputs maintain a consistent state. For example, in FDA terms this is called ‘Design control’. In our view, regulatory perspectives align well on the value and importance of DESIGN INPUTs as the basis for the design of systems. Both align on the value and importance of DESIGN OUTPUTs that define the result of the design process in ways that allow the design to be validated. And, finally, both align on the value of VERIFICATION and VALIDATION showing that requirements, and ultimately the user’s expectations, have been satisfied.
This leads to follow-up questions like: What can be different with different Lifecycles? What will be the same? In the end, the documentation might be produced differently using agile principles and practices, but the finished product might not look any different than it did before Agile was introduced.
Speakers:
Peter Pedross
is CEO and founder of PEDCO AG. As being a very early certified SAFe SPC’s, Mr. Pedross has been working with SAFe since 2011 and gained first experiences with Agile (XP) since 1999.
Besides his degree as a software engineer, he also studied finance and management psychology. Prior to founding PEDCO AG, Peter Pedross was responsible for life cycles, processes, methods, and tools at a leading Swiss financial institution and other companies in the technical and regulated sector.
On a part-time basis, he is also President of the Board of Directors for Information Technology of the Swiss Association of Quality (SAQ), a member of the Board of Directors and leader of the Special Interest Group ‘Lean & Agile Organizations’ at SAQ.
Thomas Karl
Thomas is a Lean-Agile-QMS pioneer. As Enterprise Agile Coach and Advisor, he helps clients to harness the power of disruption with Lean Quality Management and Quality Engineering. He is a large-scale, lean-agile, program and C-Level Coach, a SAFe® Summit and KeyNote speaker. He supports industry leading clients in large-scale distributed agile transformations and process improvement projects.
He had several leadership roles including leading teams and projects at Accenture and at multiple clients. He is well known for building and rebuilding (turnaround) high performing teams and organizations. He developed and managed complex business strategies that have yielded positive returns on investment.
.
.
Read one of our case studies:

Lean Process Transformation in a MedTech Organization
Send download link to:

Being Agile AND Regulatory Compliant at a Large Insurance Company
Send download link to:

Lean Process Transformation in the Automotive Industry
Send download link to:

Instantiation and Tailoring in a Digital Transformation
Send download link to: