To many organizations, compliance is an essential part of operations. It’s important that the employees follow the laws, regulations, and standards as they are following their everyday processes and building large solutions.
Many of these organizations which include medical devices, automobiles, banking and financial services, and defense, are looking to adopt and/or scale agile programs with the goal of introducing best practices to be a lean organization all while staying compliant. Many enterprises are subject to other regulations, such as, ASPICE, HIPAA, ISO 26262, CMMI, IEC 61508, that require similar attention and audits to ensure compliance.
Historically, organizations operating under such regulations have relied on comprehensive quality management systems (QMS). Continually addressing compliance concerns is one of the eight practices of SAFe’s Business Solution and Lean Systems competency.
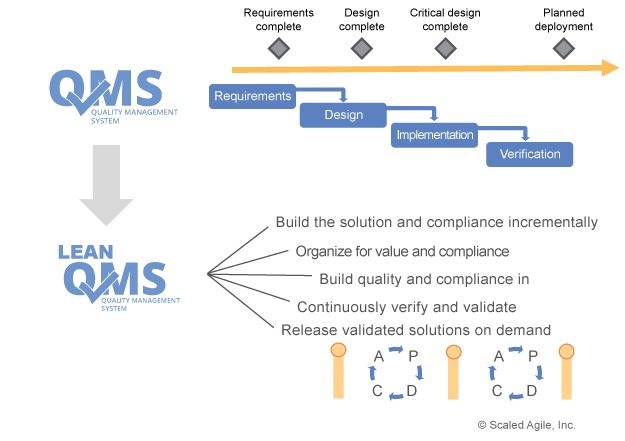
Unfortunately, many QMS systems are heavily influenced by traditional phase-gated waterfall methods. This makes it harder or even impossible for organizations to adopt new methods such as agile because they are used to the way they are working and don’t want to change that. On the right, you will see how SAFe describes an incremental approach to both development and compliance.
This video shows how to establish compliance while working in an agile manner and following the Scaled Agile Framework and using Applied SAFe.
Noncompliant organizations usually face monetary fines and penalties and can have multiple on-site compliance audits and inspections by regulatory agencies. In addition to this, brand reputation can also be damaged by companies that experience repeated — or particularly glaring — compliance breaches.
One of the most important things to remember is that your employees should not have to worry about compliance. They should follow their processes, and as they follow their processes they will automatically be compliant.
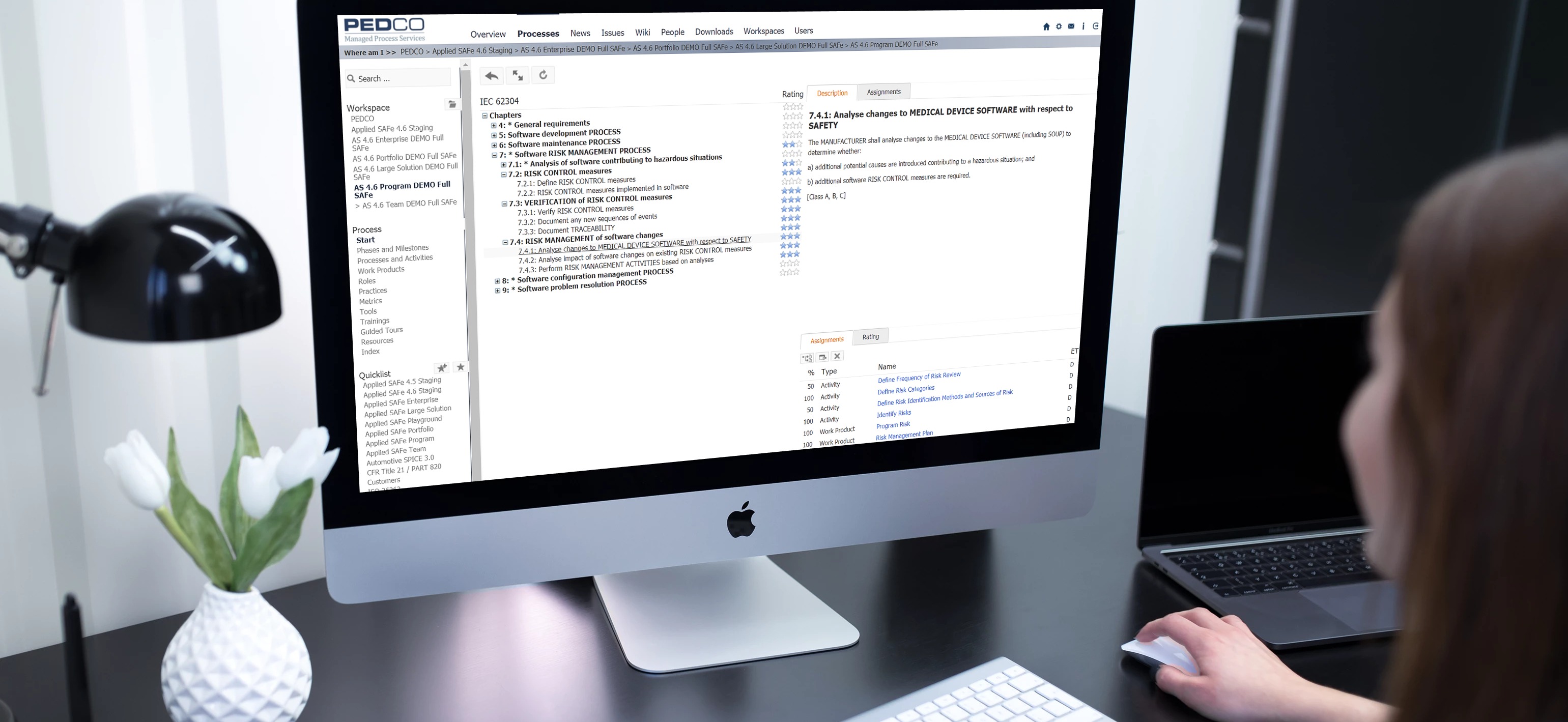
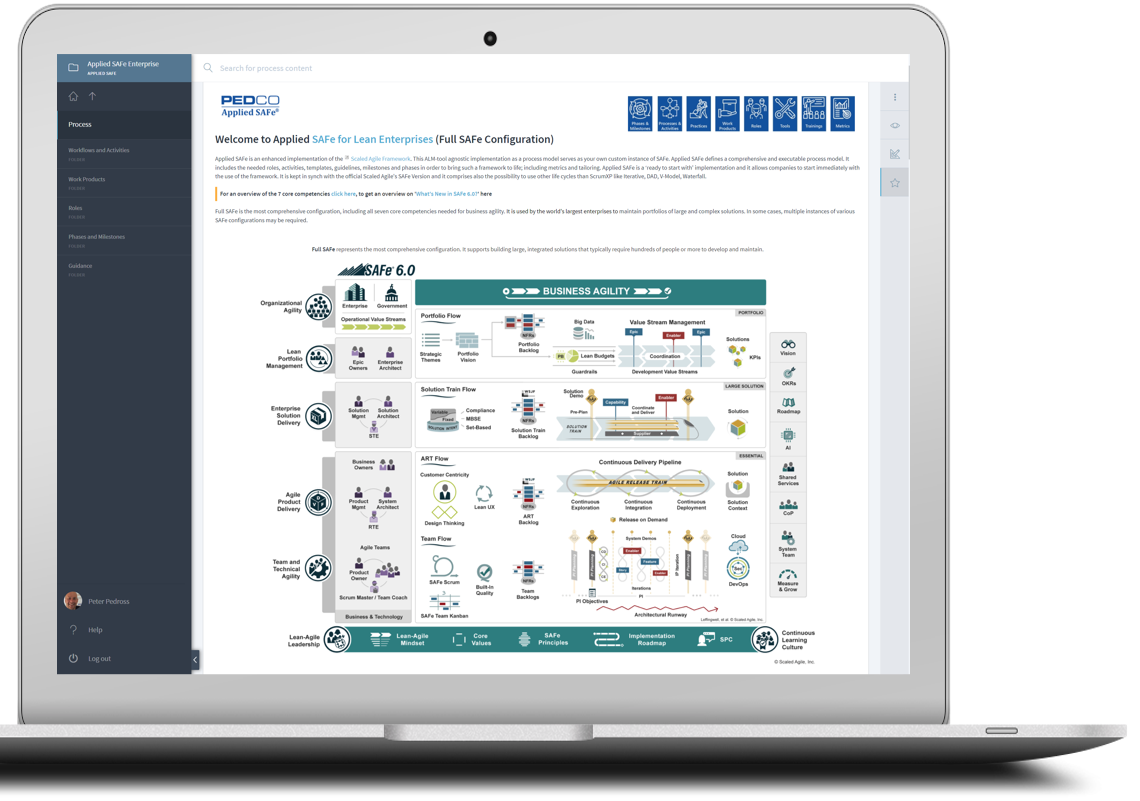
With Applied SAFe we created a Scaled Agile approved solution to fulfill requirements from various reference models like CMMI, Automotive SPICE 3.0, IEC 62304, CTR-180 (FDA) and others. Applied SAFe can further include and ensure compliance with other Reference Models like HIPPA or your own company-specific references models.
Be agile, efficient and compliant to regulatory requirements within organizational processes and even in your tailored instances of Portfolio, VS, Programs, and Teams.