Are you sure that your software projects comply with all the applicable guidelines and regulations?
Regulatory and organisational requirements are becoming ever more demanding. Project productivity should be enhanced and the quality standards adhered to.
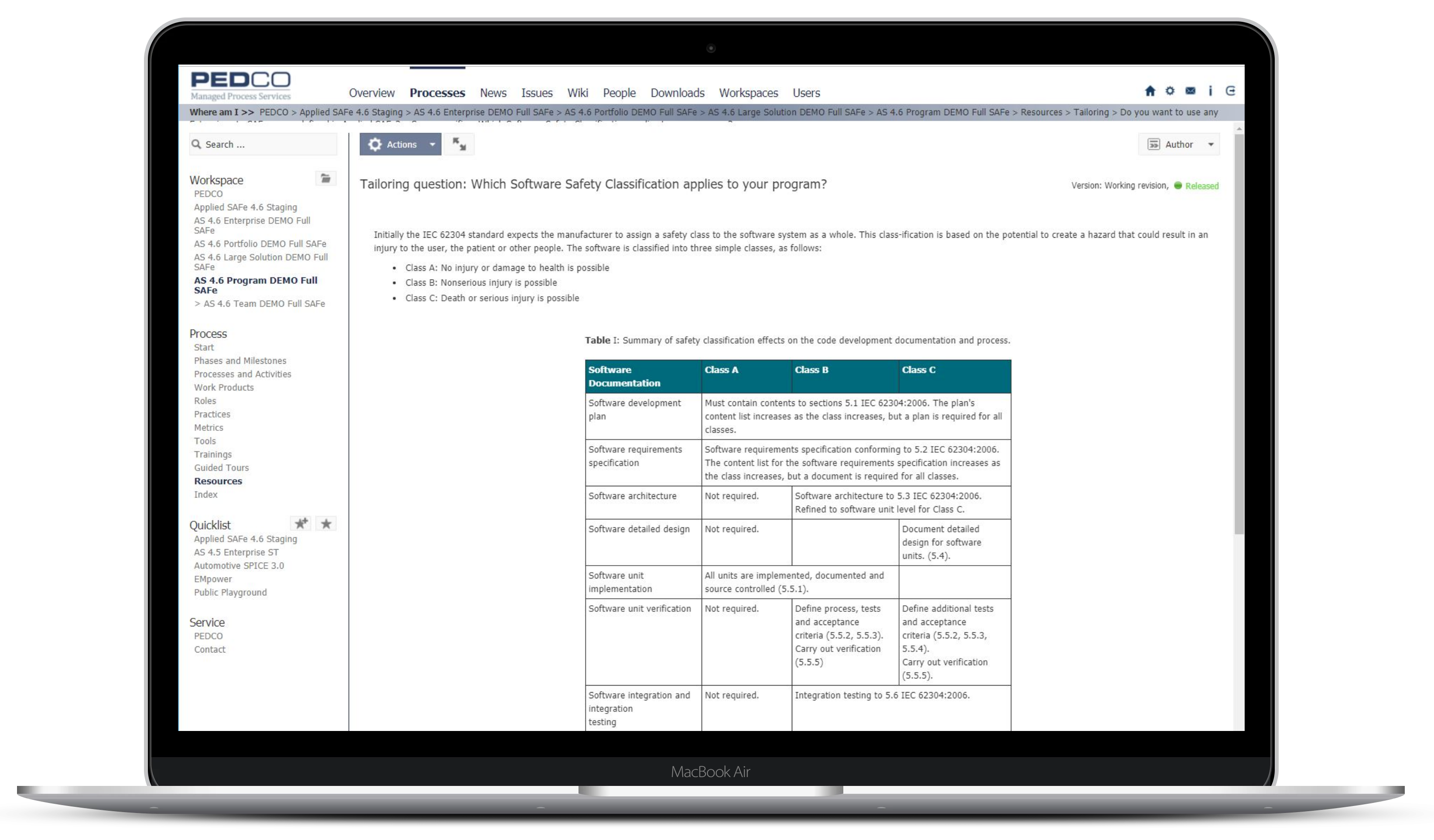
Organizations must demonstrate that their system meets its intended purpose and has no unintended consequences that might cause harm.
Read our MedTech Case Study
Send download link to:
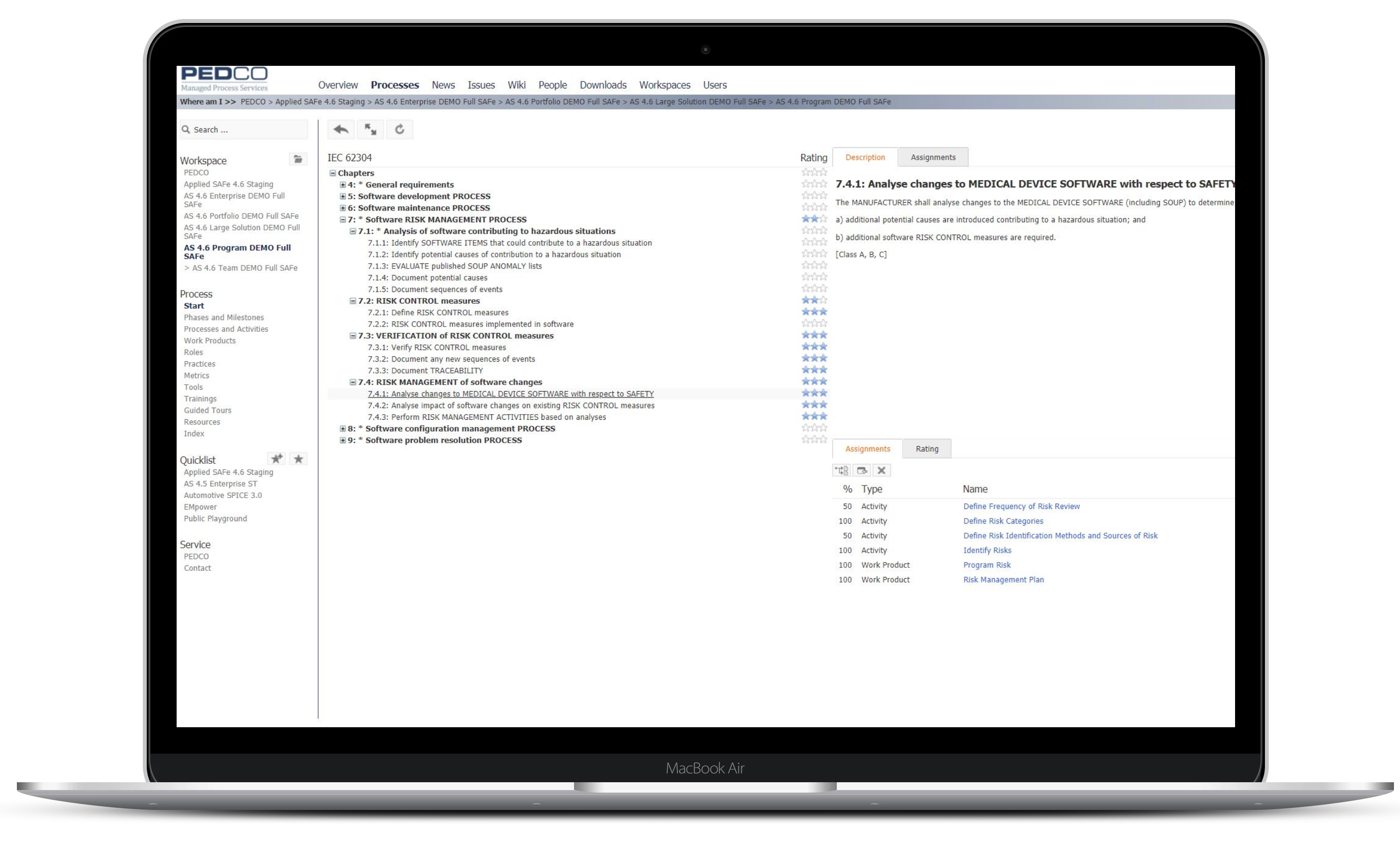
Ensure compliance with various reference models like IEC 62304, ISO 13485, ISO 14971 and ‘FDA CFR, Title 21, Part 820.30’ or your own company-specific reference models.
If you do not want to do this customization by yourself, we will help you to adapt the standard process implementation to your needs.
Read what others have to say
“When Peter presented PEDCO’s Applied SAFE demo I immediately realized the potential and benefit for companies. This product uniquely combines process modeling with a state of the art solution for scaling agile. That’s why I accepted to be a member of the PEDCO Advisory Board. As co-author of the TIR45 ‘Guidance on the use of AGILE practices in the development of medical device software’ I want to stress that agile definitely can be used for the development of medical device software and does bring value by enhancing customer focus and efficiency.”
“Medical device companies are often overwhelmed with regulatory requirements for their complex devices. With Applied SAFe® these companies can not only prove that the development of complex physical products using SAFe® still meets all regulatory requirements but also have a very performant tool to support a sustainable SAFe® implementation. Together with our products, customers will almost immediately be able to develop medical device products.”
“When we discovered Applied SAFe® we were relieved! This solution makes it possible for us to adopt SAFe® to deal with the complexity within our organization, even though it’s regulated and has compliance challenges. This platform helps our organization reduce waste and do what is really needed, which allows us to have lean processes so we can bring our product to market.”
Do you want to ensure compliance with your processes?
Error: Contact form not found.